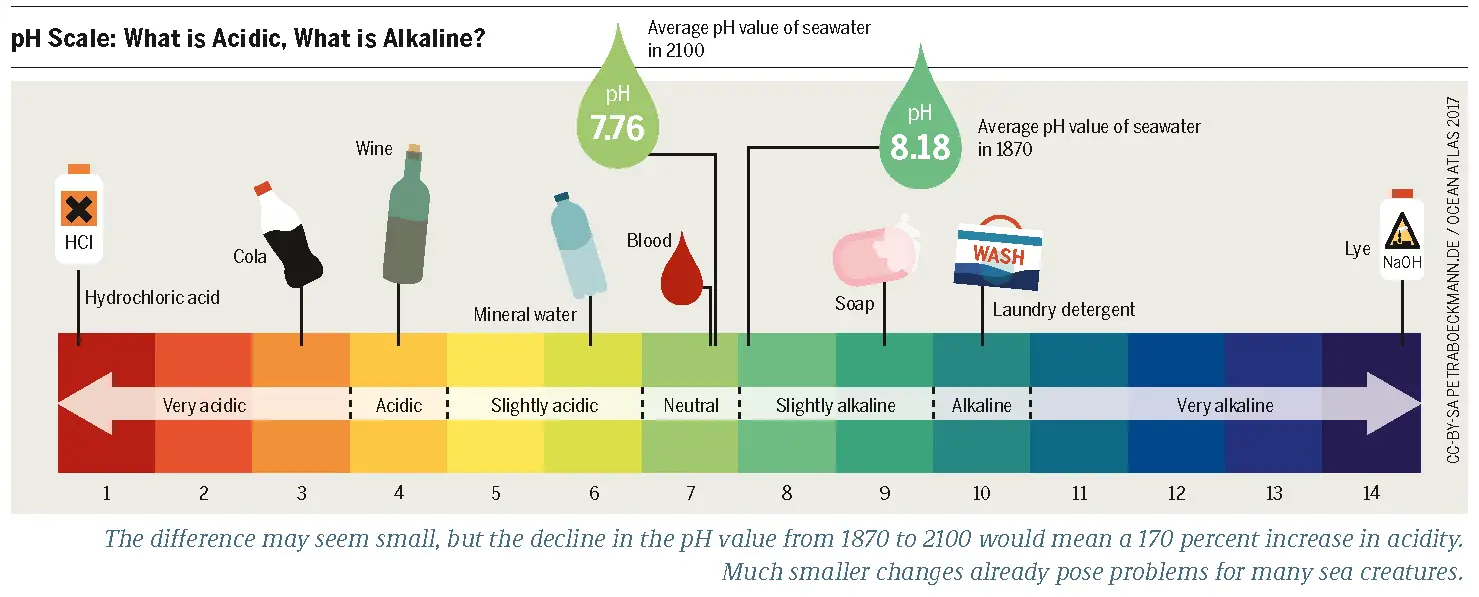
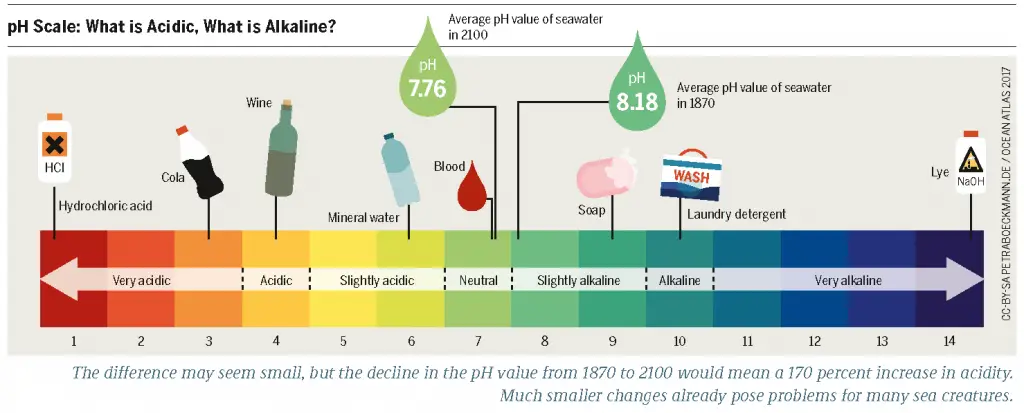
The pH of most pesticides ranges between 5.5 and 7. Therefore, most pesticides are slightly acidic and they reduce soil pH which is typically between 6.5 to 7.
Pesticides are significant for controlling diseases, pests, and other factors that are harmful to plants. However, they also come with several disadvantages. A common phenomenon associated with pesticides is the alteration of soil pH. Typically, an ideal soil pH ranges between 6.5 and 7.0. This is known as a neutral pH.
Most plants grow well under neutral soil conditions. The pH of most pesticides ranges between 5.5 and 7. Therefore, most pesticides reduce soil pH. This means they make the soil more acidic, mainly if applied in excess. Judging by international agricultural statistics, most pesticides are soil contaminants.
Effects of Pesticides on Soil pH
Excess or long-term use of pesticides deprives the soil of essential nutrients such as magnesium and calcium. This is due to an increase in soil acidity. The most common soil pollutants in pesticides include toxaphene, heptachlor, DDT, chlordane, endrin, and hexachlorobenzene.
Such compounds can bio-magnify up to 70,000 times their original concentrations. With time, the soil becomes too acidic that it cannot support the life of relevant organisms, such as earthworms and free-living bacteria that assist in nitrogen-fixing.
On the other hand, pesticides that are too alkaline can alter the soil pH by reducing the concentration of iron, zinc, and manganese. However, excess use of alkaline pesticides is rare.

Effects of Alkaline Water on Pesticides
The standard pH of water is 7, which means neutral standard. In other words, water with a pH of seven has an equal number of hydrogen ions (H+) and hydroxyl ions (OH-). Most pesticides are effective when mixed with water that is slightly acidic or neutral. Alkaline water refers to water that has a pH of above 7.
This is an important difference because a PH of 7.5 is about ten times more alkaline than that of 7. Most times, alkaline water reduces the effectiveness of pesticides. The reason behind the deprivation is the occurrence of alkaline hydrolysis.
Alkaline hydrolysis, in turn, degrades pesticides by rendering them non-toxic. This happens through the dissociation of pesticide molecules which leads to the release of electrically charged atoms.
Individual ions that are polarized tend to reassemble with other ions. When this happens, the original forms of pesticides become altered. Therefore, the insecticide will have no insecticidal or miticidal effects. The effectiveness of pesticides depends on their toxicity, thus their ability to poison pests and weeds.
To avoid such occurrences, pesticide manufacturers have details concerning the effect of water pH on pesticide half-life. The half-life of a pesticide refers to the time required for 50% of the compounds in the pesticide to hydrolyze.
The best strategy to avoid alkaline hydrolysis is to test water PH before using it to mix pesticides. Using a litmus paper is beneficial as it provides accurate results. The blue color on the piece of paper indicates alkaline water; red color indicates an acidic solution while purple represents a neutral solution.
Best Water pH for Spraying Pesticides
As mentioned earlier, the quality of water affects the performance of herbicides. Water pH quality depends on several factors. They include hardness, temperature, saltiness, and presence of organic matter, among others. Poor water quality can have several adverse effects on pesticide application.
For instance, water with high pH causes hydrolysis which renders pesticides inactive. High water temperatures accelerate the process of dissociation of pesticide molecules. Hard water contains compounds that affect oils and surfactants, thus interfering with properties of dispersion, wetting, and emulsification.
Hard or Soft Water
Soft water is the natural form of water that contains no or little minerals. The presence of calcium and magnesium at high levels leads to “hard” water.
The softness or hardness of water is measured in CaCO3 mg/L. Hard water is alkaline, therefore has a high pH. This causes hydrolysis of pesticides.
Soft water is not alkaline as it contains calcium and magnesium in low amounts. Therefore, this is the best to use for pesticide spraying is soft water. In terms of measurement, the water should be 50 CaCO3 mg/L or less.
Temperature
The higher the water temperature, the higher the rate of hydrolysis. However, this will depend on the type of pesticide used for the application. According to research by Purdue University, most herbicides work with water temperatures between 70 degrees F and 102 degrees F.
Anything above or below decreases the performance of herbicides. The majority of farmers suggest that ground and surface water should be stored in poly-tanks for hours or days so that the water can pick up the moderate temperature. Concerning the pH, it should range between 6.0 and 7.0 to prevent dissociation of pesticide molecules.
Organic matter such as leaves and algae should be minimum as it can react with some compounds due to an increase in alkalinity.
How to Mix Water with Pesticide
Getting an efficient pesticide requires proper calculations when mixing the chemical with water. To begin, check the label on the pesticide to determine the application rate. It is essential to know the concentration to use. The intensity depends on the size of the area of application.
For example, a 10 ml concentrate per 5L water is perfect for a 40 square meters area. A 5 g powder per 5L water covers an area of 20 square meters. Therefore, work out the area to be sprayed before obtaining your solution.
Next, calculate the amount of concentrate needed to cover the area. After that, calculate the amount of water required to dilute the concentrate.
While mixing your solution, consider the rules that apply in obtaining a pesticide. First, you should work in an open area as the fumes produced while mixing are toxic when inhaled.
Also, put on the appropriate protective equipment as indicated on the pesticide label. Most pesticides require that you wear a respirator. The mixing container should be large enough to accommodate the required chemical amount.
Remember to use a clean container to avoid contaminating the chemical as a dirty container will affect the pesticide’s efficacy. Manufacturers recommend that you put a small amount of water in the container first, add the concentrate then shake.
This is to ensure even distribution of the chemical, after which you can add the rest of the water. The last step is stirring the solution with a flat paddle. Consider using steel, aluminum, or plastic paddles as they are impervious.
Best Time for Spraying Pesticides
Most people would want to spray pesticides right after they see the destructive pests. That may work, but not as effective as expected. There are several rules which apply when it comes to timing. The best time is when an insect is at the most active stage of development.
That said, you should pay attention to the manufacturer’s instructions regarding application timing. Also, timing depends on the type of product used for the application. It all comes down to using the products during the most favorable weather and an insect’s time of metamorphosis.
Therefore, you have to be knowledgeable about the pesticide you are using and the insect you want to eradicate.
Weather
Pesticides can be dangerous when applied during rain. This is because the concentrates are washed down the streams and other water bodies, thus endangering aquatic life, human beings and animals that drink from the water sources.
Hence, the best time to use your pesticide is when the soil is moderately dry, and there is no sign of rain. Also, consider spraying a pesticide when the air is cool, that is, early in the morning and evening. Too much heat during the day causes most pesticides to burn plants.
Half-life of Pesticides
The half-life of a pesticide refers to the time taken for a pesticide’s efficacy to reduce by half. In a single half-life cycle, a pesticide breaks down by 50%. After two half-lives, the efficiency of a pesticide will be 25%. Pesticides have different half-lives depending on environmental conditions. Some pesticides may have a half-life of fewer than 16 days while others may have half-lives of one to two years.
Factors that Influence Half-Life
Sunlight
Sunlight breaks down some chemical bonds of pesticide molecules, thus producing breakdown products.
Water
Water causes hydrolysis by dissociation of pesticide molecules. However, the process of hydrolysis is slow.
Microbes
Bacteria and fungi are organisms involved in breaking down compounds, including those found in pesticides.
Sorption
This is the process whereby some chemicals bind tightly to particles and move away with the particles, thus reducing pesticide half-life.
PH of Pesticides
Herbicides
The appropriate pH range for herbicides is 3 to 6. They are known as weak acids. Such acids partially dissociate when mixed with water.
Atrazine
This is a triazine herbicide used for controlling weeds in corn and sorghum. It has a PH value of 5.5.
Molluscicides
Molluscicides are agents that destroy mollusks such as snails. Their pH range from 5.0 to 6.5.
Metaldehyde
This is a popular molluscicide with a PH of 5.4.
Rodenticides
Rodenticides are pesticides that eradicate rodents. The appropriate pH for rodenticides is between 4.0 and 4.3.
Bromethalin
Bromethalin is one of the top five rodenticides used by farmers. Having a PH of 4.0, it works by interfering with oxidative phosphorylation in the brain and liver of rodents, thus causing brain swelling and death.
Miticides
They are chemicals that kill mites. The ideal pH range for most miticides is 5.0 to 7.0.
Azadirachtin
Azadirachtin is a perfect example of a miticide with a pH of 5.0.
Insecticides
Insecticides are organic, inorganic or natural pesticides that eradicate insects. Their ideal pH range is 5.0 to 7.0. However, several insecticides at or above pH 7.0.
Dieldrin
This is a common insecticide with a pH value of 6.8.
Foggers
These are pesticide products that aerosol propellants which release chemical fogs at once to fumigate an infested area. The pH value of chemicals in the foggers depends on the type of pest being eradicated.
Fungicides
Fungicides are pesticides that kill and prevent the growth of fungi and their spores. The optimum PH range for fungicides is 4.0 to 6.5.
Bonide 811 Copper 4E Fungicide 160Z
With a pH of 6.5, it controls plant diseases such as downy mildew, black spot, and powdery mildew.